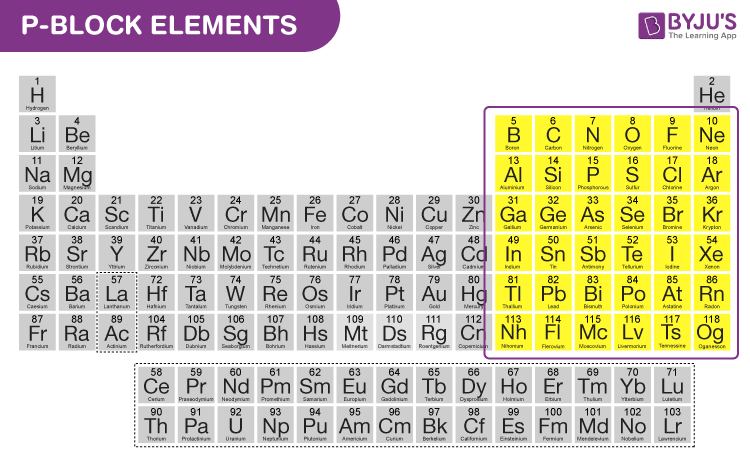
P block elements are those in which the last electron enters any of the three p-orbitals of their respective shells. Since a p-subshell has three degenerate p-orbitals each of which can accommodate two electrons, therefore in all there are six groups of p-block elements.
P block elements are shiny and usually a good conductor of electricity and heat as they have a tendency to lose an electron. You will find some amazing properties of elements in a P-block element like gallium. It’s a metal that can melt in the palm of your hand. Silicon is also one of the most important metalloids of the p-block group as it is an important component of glass.
In this section, we will study the elements of P-block and their properties.
P block elements consist of

P block elements are nothing but the element in which the last electron enters the outermost p-subshell. P block starts from the 13 th group and goes till the 18 th group in the periodic table.
You must have seen that coal is used in villages to cook food. It is nothing but a P-block element i.e. carbon. Diamonds used for making beautiful ornaments are also made up of carbon. Aluminium foil made up of aluminium is also made up of the p block element.